Draw the different resonance structures of the naphthalene & show how each one show a conjugated bond pair for it to be aromatic compound?
In case of multiple questions within a query, please post each question individually and let us know where you are getting stuck so that we would be able to explain things better.
Solution for your first query,
Resonance structures of naphthalene are as follows:
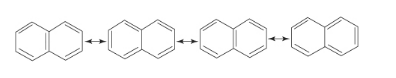
Conjugated double bonds in a molecule means alternate single and double bonds. These enable the electrons to be delocalized over the whole system and so be shared by many atoms. This means that the delocalized electrons may move around the whole system.
In each resonance structure of naphthalene molecule, we can see that there are alternate single and double bonds that means conjugated bond system.