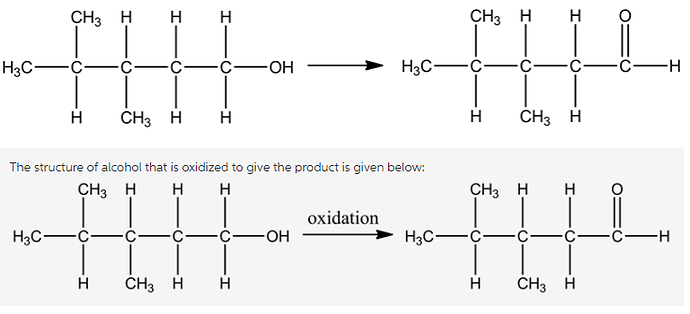
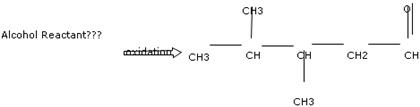
Draw the alcohol that is oxidized to give the product shown below. Show all hydrogen atoms.
Concepts and reason
In organic chemistry, retrosynthetic analysis is one of the techniques that is used to determine the structure of reactant by analyzing key bonds in the structure of product. Alcohols are compounds with (O - H) functional group. Alcohols are oxidized to carbonyl compounds as well as carboxylic acids depending on the reagent used for oxidation.
Fundamentals
Retrosynthesis: Retrosynthetic analysis is used for designing the synthesis of organic molecule. In this analysis, planning of organic synthesis takes place by disconnecting the target molecules into precursors.

Answer:
The structure of the organic molecule is given below:
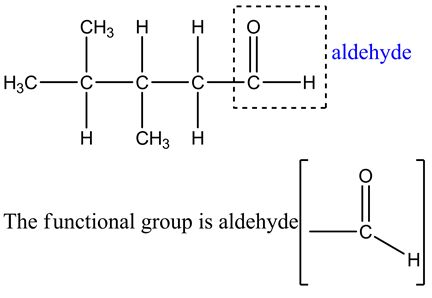
Explanation:
The functional group present in the given organic compound at one of the terminal carbon atom is aldehyde. There is double bond between oxygen and carbon.
The structure of the primary alcohol is given below:
Explanation:
The functional group (-OH) is present on the compound and the primary alcohol undergoes oxidation using specific reagent to give aldehyde molecule.