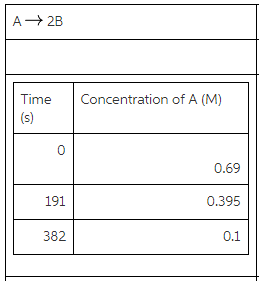
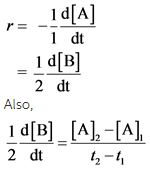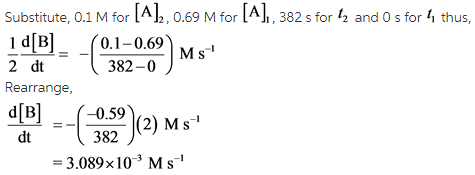Determine the average rate of change of B from t=0 s to t=382 s.
Concepts and reason
This is based upon the concept of chemical kinetics.
Rate of the reaction tells how quickly or slowly a reaction will take place. It also tells how quickly or slowly a reactant will disappear or a product will be generated.
Fundamentals
Average rate of a reaction is expressed in terms of reactant and product as follows:
Consider a reaction:
![]()
Here, a, b, x and y are the stoichiometric coefficients of P, Q, M and N respectively. P and Q are the reactants and M and N are the product of the reaction.
Rate of reaction ® is defined as the rate of disappearance of reactant or rate of production of products. So, Mathematically, it is represented as
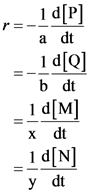
Average rate of reaction is represented as,

Answer:
For the reaction as follows:
![]()
Expression for average rate, r is as follows
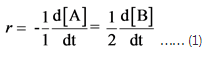
Expression (1) is as follows: