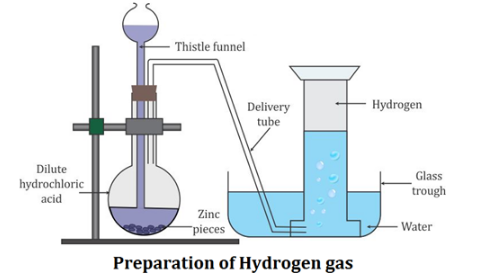
describe the preparation of hydrogen in the laboratory
Laboratory Preparation of Hydrogen:
Hydrogen is prepared in the laboratory by the action of the dilute hydrochloric acid or dilute sulphuric acid on granulated zinc.
Use of Granulated Zinc
Granulated zinc contains an impurity like copper which acts as a positive catalyst. A positive catalyst increases the rate of a chemical equation. This is the reason why granulated zinc is preferred over pure zinc for the laboratory preparation of hydrogen gas.
Reaction:
Metal + Dilute acid → Salt + Hydrogen
Zn + 2HCl → ZnCl2 + H2 ↑
Zn + H2SO4 → ZnSO4 + H2 ↑
Collection of Gas : Hydrogen gas is collected by downward displacement of water.