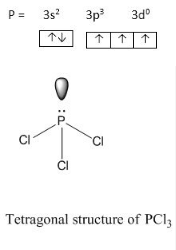
a) describe the hybridisation in case of PCL3
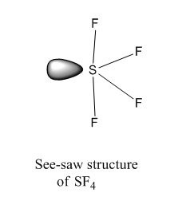
b) reduce the shape of SF4 molecule on basis of VSEPR theory
- PCl3 :
Phosphorous has 3 electrons in its outer most shell and one lone pair so it tetravalent in nature, it can join to3 other Cl atoms and get sp3 hybridisation and tetrahedral shape.
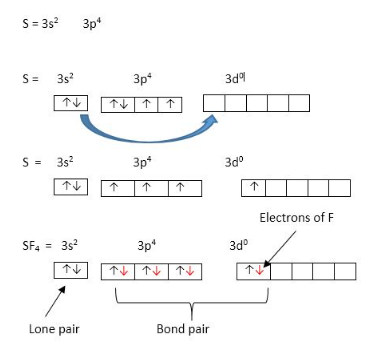
- SF4 :
S has 6 valence electrons
F has 7 valence elctrons hence 4 X 7 = 28 electrons