a) Describe the following giving suitable examples:
(i) Cannizzaro reaction (ii) Aldol condensation
b) Give a chemical test to distinguish between ethanal and propanal.
(a) (i) Cannizzaro reaction: Aldehydes having no α-hydrogen atoms undergo self oxidation and reduction (disproportionation) reactions on treatment with a concentrated alkali. In such reactions, one molecule of aldehyde gets oxidised to form an acid and the other molecule of aldehyde gets reduced to form an alcohol.
For example, two molecules of formaldehyde, in the presence of concentrated NaOH, produce methanol and sodium formate.
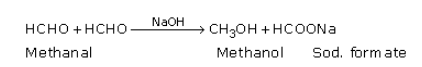
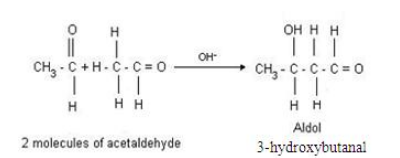
(ii) Aldol condensation: In the presence of a dilute alkali, aldehydes and ketones (having at least one α-hydrogen atom) produce β-hydroxyl aldehyde (aldol) and β-hydroxyl ketone respectively. The aldol and ketol then readily lose water to give α, β-unsaturated carboxyl compounds. Such reactions are called aldol condensation.
For example, in the presence of dil. NaOH, ethanal (aldehyde) produces 3-hydroxybutanal (aldol), which then readily loses water to produce but-2-enal.
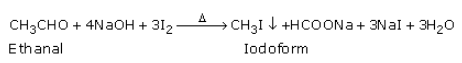
(b) When warmed with iodine and sodium hydroxide solution, ethanal gives yellow crystals of iodoform.
Propanal does not give this iodoform test