Describe Rutherford’s scattering experiment.
Rutherford’s Scattering Experiment
- Rutherford selected a gold foil as he wanted a very thin layer.
- In his experiment, fast moving alpha particles were made to fall on a thin gold foil.
- Alpha particles are helium ions with +2 charge and have a considerable amount of energy.
- These particles were studied by means of flashes of light they produced on striking a zinc sulphide screen.
- He expected the alpha particles to pass through the gold foil with little deflections and strike the fluorescent screen.
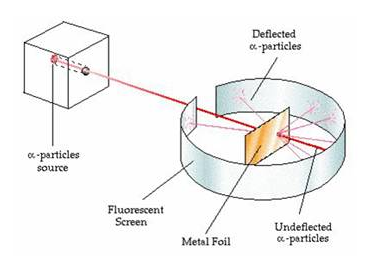
But the observations he made were quite unexpected.
- He observed that most of the alpha particles passed straight through the gold foil.
- Some were deflected through small angles and some were deflected through large angles.
- Very few appeared to bounce back.
From the experiment he concluded that-
- As most of the alpha particles passed through the gold foil without getting deflected, most of the space inside the atom is empty.
- Very few particles deflected from their path; this indicated that the positive charge of the atom occupies very little space.
- A small fraction of alpha particles bounced back by 180 degrees, this indicated that the entire positive charge and mass of the atom was concentrated in a very small volume within the atom.
- Based on his observations, he formulated his ‘Theory of atom’.
The main features of Rutherford’s Theory of an Atom
- There is a positively charged centre in the atom called the nucleus in which nearly all the mass of the atom is concentrated.
- Negatively charged particles called electrons revolve around the nucleus in paths called orbits.
- The size of the nucleus is very small as compared to the size of the atom.
- His model can be compared to the solar system where the planets are compared with electrons and the sun with the nucleus.