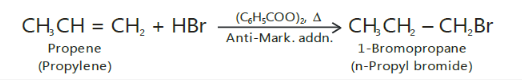describe Markovikov rule and kharash effect
Markovnikov’s rule .
Markovnikov, a Russian chemist, studied a large number of such addition reactions and postulated an empirical rule in 1869 which is known after him as Markovnikov’s rule.
The rule states that,
“The addition of unsymmetrical reagents such as HX, H2O, HOX, etc. to unsymmetrical alkenes occurs in such a way that the negative part of the addendum (i.e., adding molecule) goes to that
carbon atom of the double bond which carries lesser number of hydrogen atoms.”
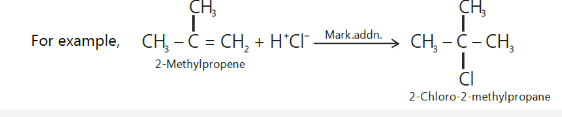
Kharasch effect
It should be noted that Markovnikov’s rule is not always followed.
In the presence of peroxides such as benzoyl peroxide, the addition of HBr (but not ofHCl or HI) to unsymmetrical alkenes takes place contrary to Markovnikov’s rule.
This is known as Peroxide effect or Kharasch effect.