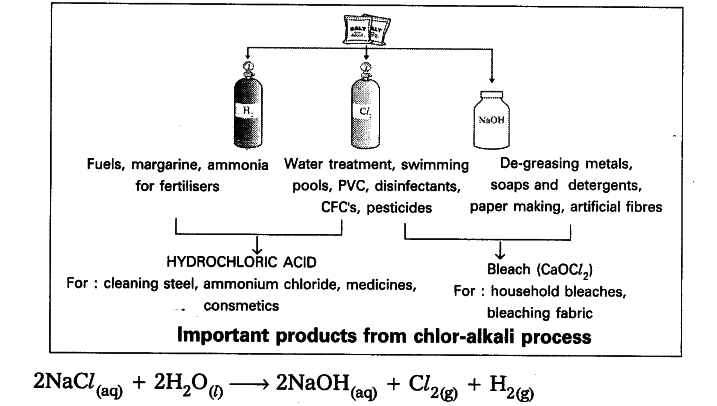
- When electricity is passed through an aqueous solution of sodium chloride . (called brine), it decomposes to form sodium hydroxide.
- The process is called chlor-alkali process - because of the products fromed chlor for chlorine and alkali for sodium hydroxide.
- Chlorine gas is liberated at the anode and hydrogen gas at the cathode.
- Sodium hydroxide solution is formed near the cathode.
- The three products produced in this process are all useful.