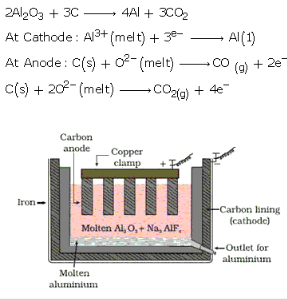
Describe Hall-Heroult process used for the electrolysis of pure alumina?
n Hall-Heroult process, purified Al2O3 is mixed with Na2AlF6 or CaF2 which lowers the melting point of matrix and brings conductivity. The fused matrix is electrolysed. The cathode and anode is made of steel and graphite respectively.
The various reactions which take place at cathode and anode are: