Derive the relation between molarity and molality
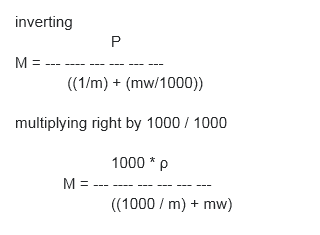
M = molarity = moles solute / Liter solution
m = molality = moles solute / kg solvent
ρ = density = g solution / mL solution = kg solution / L solution
mw = molecular weight (molar mass) of solute in g/mole
Equations:
(1) M = moles solute / L solution
(2)m = moles solute / kg solvent
(3) ρ = kg solution / L solution
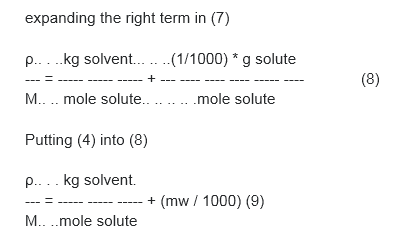
(4)mw = g solute / mole solute
(5) kg solution = kg solvent + kg solute
multiplying (3) x 1/(1) Equations:
(1) M = moles solute / L solution
(2)m = moles solute / kg solvent
(3) ρ = kg solution / L solution
(4)mw = g solute / mole solute
(5) kg solution = kg solvent + kg solute
multiplying (3) x 1/(1)