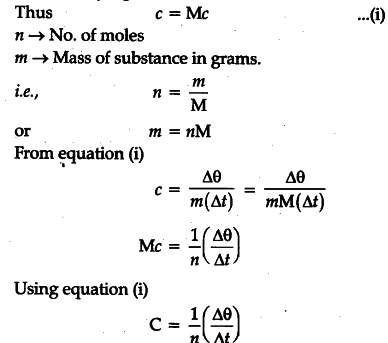
Molar specific heat of a substance is defined as the amount of heat required to raise the temperature of one gram mole of the substance through a unit degree. By definition one mole of any substance is a quantity of the substance whose mass in gram is numerically equal to the molecular mass M.