Decide whether each of the following reaction involves oxidation-reduction. If it does, identify what is oxidised and what is reduced?
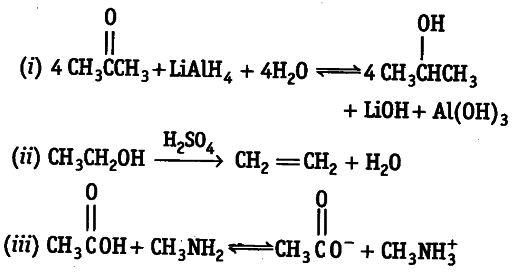
(i) In this redox reaction, H in LiAIH4 gets oxidised because of the addition of oxygen atom that leads to the formation of OH-. Propanone gets
reduced because of addition of hydrogen atom to 2-propanol
(ii) This is not a redox reaction as neither hydrogen or oxygen or e is removed or added.
(iii) This is not a redox reaction as neither hydrogen or oxygen or e is removed or added.