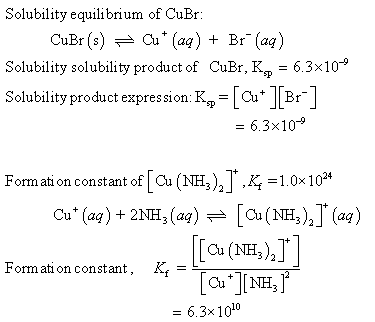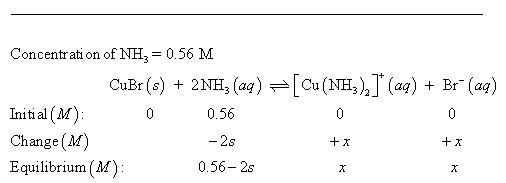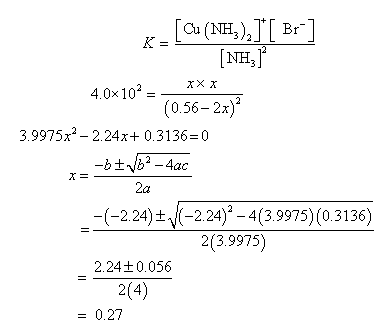Copper(I) ions in aqueous solution react with NH3(aq) according to Cu^+(aq) + 2NH3(aq) —> Cu(NH3)2^+(aq) note:Kf = 6.3 x 10^10 Calculate the solubility (in g.
Answer:
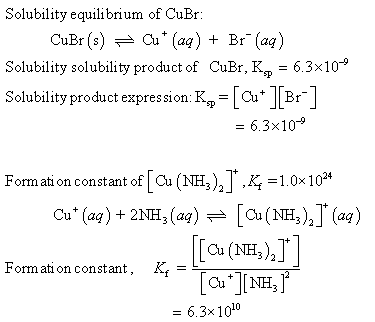
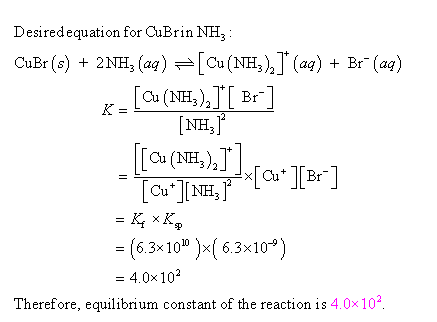
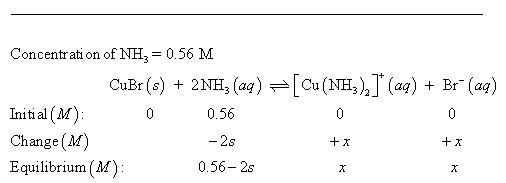
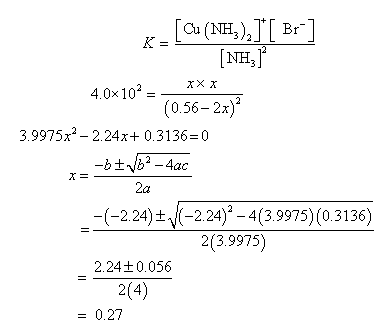
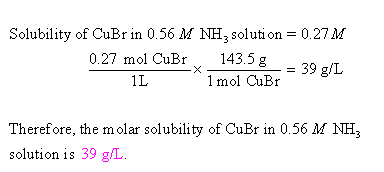
Copper(I) ions in aqueous solution react with NH3(aq) according to Cu^+(aq) + 2NH3(aq) —> Cu(NH3)2^+(aq) note:Kf = 6.3 x 10^10 Calculate the solubility (in g.
Answer: