E° value of {{Zn}^{2+}} / Zn = —0.76 V is less than that of {{Cu}^{2+}}/Cu = +0.34 V. It means that zinc is a stronger reducing agent and can easily displace the {{Cu}^{2+}} ions present in the complex.
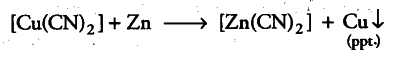
So, zinc can be isolated by hydrometallurgy only when stronger reducing agents than zinc are present. But these react with water to evolve hydrogen gas. Thus, these metals also do not solve the purpose and zinc cannot be extracted by hydrometallurgy.