Consider these two entries from a fictional table of standard reduction potentials.
**X3+ + 3e- rightarrow x(s) E = -2.00 V Y3+ + 3e- **
What is the standard potential of a cell where X is the anode and Y is the cathode?
Concepts and reason
The question is based on calculating the overall cell potential, ![]() for the given reaction. It is calculated using the following formula:
for the given reaction. It is calculated using the following formula:
![]()
To calculate ![]() , the half-cell reactions with the overall reaction must be determined.
, the half-cell reactions with the overall reaction must be determined.
Fundamentals
Overall cell potential ![]() : It is the cell potential of the overall net reaction. It is calculated as follows:
: It is the cell potential of the overall net reaction. It is calculated as follows:
![]()
Here,![]() is the standard reduction potential of the reaction occurring at cathode; and
is the standard reduction potential of the reaction occurring at cathode; and ![]() is the standard reduction potential of the reaction occurring at anode.
is the standard reduction potential of the reaction occurring at anode.
While writing the half-cell reaction, oxidation occurs at anode and reduction occurs at cathode.
The overall reaction is written by combining both the half-cell reactions.
Answer:
Consider the given half-cell reactions occurring at different electrodes:
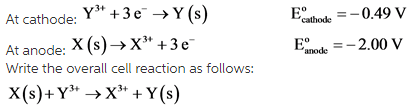
It is given that X is the anode and Y is the cathode for the half-cell reactions. Moreover, oxidation reaction occurs at anode and reduction reaction occurs at cathode.
Therefore, the half-cell reaction of anode will be reversed but, its standard reduction potential will remain same.
The overall cell reaction is written by combining both the half-cell reactions and cancelling the common electrons present in them.
Calculate the overall standard cell potential as follows:
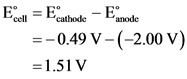
Standard cell potential,![]() , for the given reaction is 1.51 V.
, for the given reaction is 1.51 V.
Substitute the values of standard reduction potentials of cathode and anode in the given formula to calculate the value of overall cell potential.