Consider the reaction described by the following equation.
C2H4Br2(aq)+3I^-(aq) -----> C2H4(g)+2Br^-(aq)+i(negative charge)(three subscript) (aq)
The rate law is
rate = k[C2H4Br2] [I^-] Where k=4.77x10^-3 M^-1 s^-1
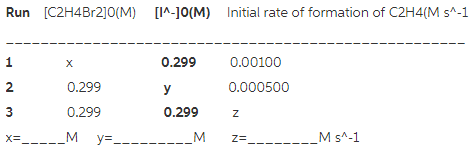
What are the missing entries in the following table?
Concepts and reason
The concept used to solve this problem is based on how rate of a reaction is dependent on several factors in chemical kinetics. According to the Law of mass action the rate of a reaction is proportional to the concentration of the reactants.Given the rate law for the reaction, each missing part can be obtained by substituting the other known values in the rate law.
Fundamentals
Rate law is an equation which relates the rate of a reaction and the concentration of the reactants. For a general reaction,
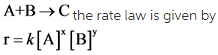
Where,[A] and [B] are concentrations of the reactants; x and y are the orders of reaction and k is the reaction constant.
Answer:
Part a
Given that the rate law of the mentioned reaction is

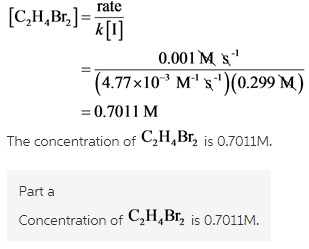
For the first run, the concentration of iodide and rate of formation are known. From these known values, unknown concentration of ![]() is calculated using the rate law.
is calculated using the rate law.
Part b
Rearrange equation (1) for [1]
![]()
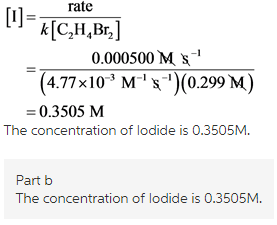
Part c
Rearrange equation (1) for [1]
![]()
