Consider the following reaction at 298 K:
2C (graphite) + O2 (g) >>> 2CO (g) Delta H = -110.5 kJ
Calculate:
- Delta S(sys) J/K
- Delta S (surr) J/K
- Delta S (Univ) J/K
Answer:
The given reaction is as follows:
![]()
Calculate the entropy change of the system as follows:
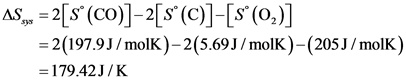

Calculate the entropy change of the surroundings as follows:
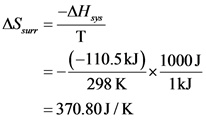
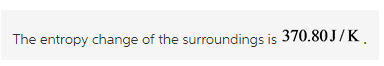
Calculate the entropy change of the universe as follows:

