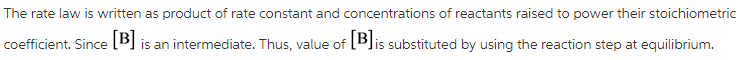#1 Consider the following mechanism:
Step 1: 2A ----> B slow
Step 2: B + C ----> D fast
Overall 2A + C ----> D
Determine the rate law for the overall reaction (where the overall rate constant is represented as k).
#2 Consider the following mechanism:
Step 1: 2A <----> B equilibrium
Step 2: B + C ----> D slow
Overall: 2A + C ----> D
Determine the rate law for the overall reaction (where the rate constant is represented as k)
Concepts and reason
The concept used to solve this problem is based on the rate law of a chemical reaction.
In a chemical reaction, the equation that relates the rate of reaction with concentrations of reactants and constant parameter is called the rate law.
Fundamentals
For a chemical reaction in which reactants A and B react to form products,
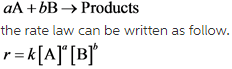
Here, [A] and [B] are concentration of A and B respectively. The rate law is determined by slowest step of the reaction. At equilibrium, rate of forward reaction is equal to rate of backward reaction.
Answer:
(1)
The elementary steps mention in question are as follow.
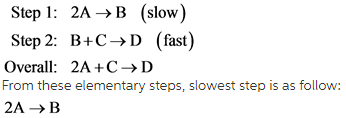
Since the rate law is written as concentrations of reactants raised to power their stoichiometric coefficient multiplied by rate constant. Thus, rate law for this reaction can be written as follow.
![]()


Explanation:
The rate law is written as product of rate constant and concentrations of reactants raised to power their stoichiometric coefficient.
(2)
The elementary steps mention in question are as follow.

Explanation:
Rate law is the product of concentrations of reactants raised to power their stoichiometric coefficient and rate constant.
From these elementary steps, slowest step is as follow:
![]()
Since the rate law is written as concentrations of reactants raised to power their stoichiometric coefficient multiplied by rate constant. Thus, rate law for this reaction can be written as follow.


Explanation: