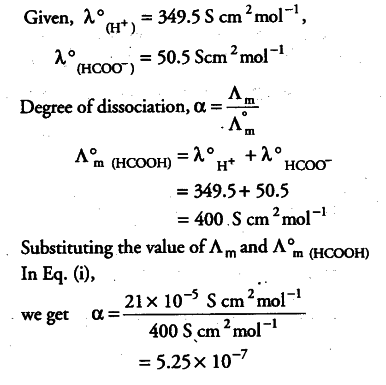Conductivity of 2.5 x ${{10}^{-4}}$ M methanoic acid is 5.25 x ${{10}^{-5}}$ S ${{cm}^{-1}}$. Calculate its molar conductivity and degree of dissociation.
Conductivity of methanoic acid = 5.25 x {{10}^{-5}} S {{cm}^{-1}}
Concentration of methanoic acid = 2.5 X {{10}^{-4}} M = 2.5 x {{10}^{-4}} x 1000 mol {{cm}^{-3}} = 0.25 mol {{cm}^{-3}}
Molar conductivity,
A_{ m } = K/C = 5.25 x {{10}^{-5}} S {{cm}^{-1}} / 0.25 mol {{cm}^{-3}}
= 21 x {{10}^{-5}} S {{cm}^{2}} {{10}^{-5}} S {{mol}^{-1}}