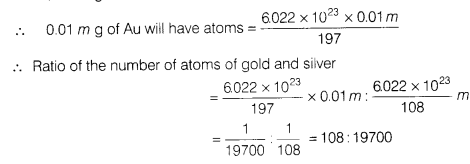A silver ornament of mass m gram is polished with gold equivalent to 1 % of the mass of silver. Compute the ratio of the number of atoms of gold and silver in the ornament.
Mass of silver (Ag) ornament = mg
Mass of gold used for polishing ![]()
Atomic mass of Ag = 108 u
∴ 1 mole of Ag = 108 g = 6.022 x 1023 atoms
Thus, 108 g of Ag have atoms = 6.025 x 1023
∴ m g of Ag will have atoms ![]()
Similarly, atomic mass of gold (Au) = 197 u
1 mole of Au = 197 g = 6.025 x 1023 atoms
Thus, 197 g of Au have atoms = 6.025 x 1023