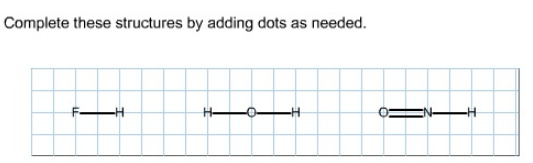
Complete these structures by adding dots as needed.
Concepts and reason
Structure of a molecule can be drawn based on valance of the atoms involved in the structure.
An atom form maximum number of bonds in order to get octet configuration.
Consider the atoms present in the structures; connect them to satisfying their valance. Place the nonbonding electrons on the atoms in the structure.
Fundamentals
Special orientation of atoms present in a molecule is called molecular structure.
Electrons that do not involved in the bond formation are called as non-bonding electrons. The on bonding electrons are generally represented with dots.
Answer:
Hydrogen forms only one bond with other atoms. Fluorine can also form a single bond to complete its octet. Fluorine belongs to Group 7A of the periodic table, its valance is 1.
Structure of is as follows:
![]()
Explanation:
Atomic number of hydrogen is 1; it has only one electron in its valance shell. So, hydrogen can form only one bond.
Atomic number of fluorine is 9; it has seven electrons in the valance shell. So, fluorine needs only one electron to get octet configuration and it has six non-bonding electrons. So, the structure can be drawn as shown below.
![]()
The dots on the fluorine atom represent non-bonding electrons.
Hydrogen forms only one bond with other atoms. Oxygen can form two bonds to get octet configuration. Oxygen belongs to Group 6A of the periodic table, its valance is 2.
Complete structure is shown below.
![]()
Explanation:
Atomic number of hydrogen is 1; it has only one electron in its valance shell. So, hydrogen can form only one bond.
Atomic number of oxygen is 8; it has six electrons in the valance shell. So, oxygen needs two electrons to get octet configuration and it has four non-bonding electrons.
So, the structure will be as follows:
![]()
Oxygen can form two bonds to get octet configuration. Oxygen belongs to Group 6A of the periodic table, its valance is 2.
Nitrogen can form three bonds to complete its octet configuration. Nitrogen belongs to Group 5A of the periodic table, its valance is 3.
Complete structure is shown below.
![]()
Explanation:
Atomic number of oxygen is 8; it has six electrons in the valance shell. So, oxygen needs two electrons to get octet configuration and it has four nonbonding electrons.
Atomic number of nitrogen is 7; it has five electrons in the valance shell. Hence, nitrogen needs three electrons to get octet configuration and it has two non-bonding electrons.
![]()