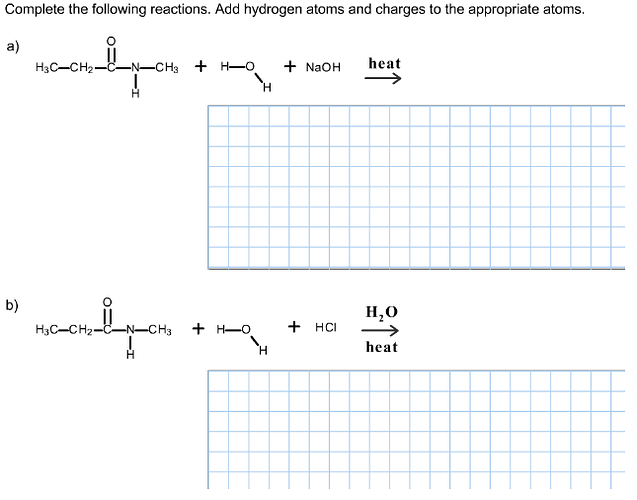
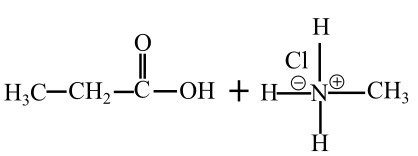Complete the following reactions. Add hydrogen atoms and charges to the appropriate atoms.
Concepts and reason
The problem is based on the concept of calculation of the hydrolysis of amide group. Hydrolysis of amide group takes place differently in acidic and basic medium.
Fundamentals
Hydrolysis of amide group in acidic medium results into formation of carboxylic acid and ammonium salt. Hydrolysis of amide group in basic medium results into formation of carboxylate ion and an amine.
Answer:
Part a
The following reaction takes place when N-methylpropanamide reacts with water in basic medium (presence of NaOH)
Part a
The products of hydrolysis of N-methylpropanamide in basic medium are shown follows:
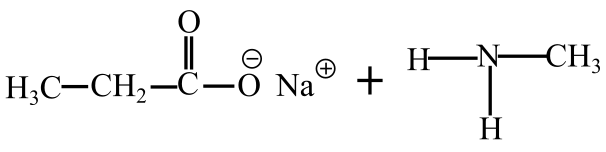
Part b
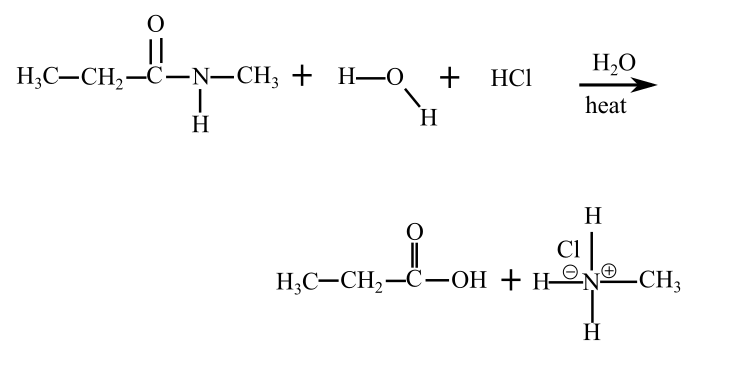
The following reaction takes place when N-methylpropanamide reacts with water in acidic medium (presence of HCl)
Part b
The products of hydrolysis of N-methylpropanamide are in acidic medium shown follows: