Compare the Stability of O2, O2(+), O2 (-), O2 (2-)
Electronic configurations:
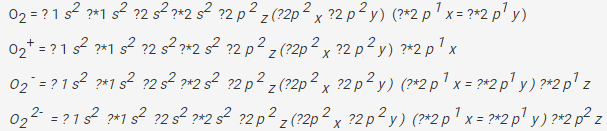
The electronic configuration of the O2+ ion containing 15 electrons can be written as:
![]()
The bond order can be found as:
B.O = (Nb -Na)/2
Nb = Number of electrons in the bonding orbitals = 10
Na = Number of electron in the anti-bonding orbitals = 5
B.O = (10-5)/2 = 5/2 = 2.5
The electronic configuration of the O2- ion containing 17 electrons can be written as:
![]()
The bond order can be calculated as:
B.O = (10-7)/2 = 3/2 = 1.5
Similarly, Bonbd order of O2 is = B.O = (10-6)/2 = 4/2 = 2
Bonbd order of O22- is = B.O = (10-8)/2 = 2/2 = 1
The higher the value of bond order, higher is the stability of the bond, so on the basis of above information, we can say that, O2+ ion is more stable than O2, O2- and O22-ions.