Compare the general characteristics of the first series of the transition metals with those of the second and third series metals in the respective vertical columns. Give special emphasis on the following points.
(i) Electronic configuration
(ii) Oxidation states
(iii) Ionisation enthalpies
(iv) Atomic size
(i) Electronic configuration The elements in the same vertical column generally have similar electronic configuration. Although, the first series has only two exceptions,
Cr = 3${{d}^{5}}4{{s}^{1}} and Cu = 3{{d}^{10}}4{{s}^{1}}$
Second transition series have five exceptions,
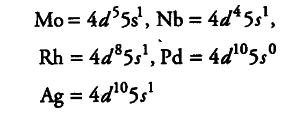
Third transition series has three exceptions,
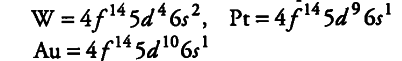
(ii) Oxidation states The elements in the same vertical
column generally show similar oxidation states. The number of oxidation states shown by the elements in the middle of each series is maximum and minimum at the extreme ends.
(iii) Ionisation enthalpies The first ionisation enthalpy in
each series generally increase gradually as we move from left to right. Though some exceptions are observed in each series. The first ionisation enthalpies of some elements in the second (4d) series are higher, while some of them have lower value than the elements of 3 d-series in the same vertical column.
(iv) Atomic size Generally, ions of the same charge or atoms in a given series show progressive decrease in radius with increasing atomic number though the decrease is quite small. But the size of the atoms of the 4d-series is larger than the corresponding elements of the 3d-series whereas those of corresponding elements of the 5d-series are nearly the same as those of 4d-series due to lanthanoid contraction.