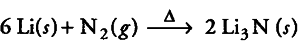Comment on each of the following observations
(i) The mobilities of the alkali metal ions in aqueous solution are
Li+<Na+<K+<Rb+<Cs+.
(ii) Lithium is the only alkali metal which forms nitride directly.
(i) Smaller the size of the ion, more highly it is hydrated and greater the hydration of the ion, lower is its ionic mobility. Since, the extent of hydration decreases in the order .
Li+ > Na+ >K+ >Rb‘+ > Cs+
Therefore, ionic mobility increases in the reverse order
Li+ < Na+ <K+ < Rb+ < Cs+
(ii) Because of its smaller size, lithium like magnesium forms a nitride while other alkali metals do not.