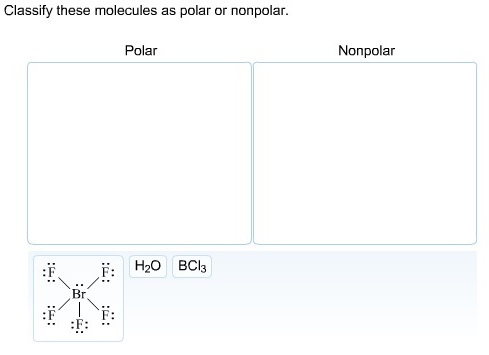
Classify these molecules as polar or non-polar.
Concepts and reason
The concept used to solve this problem is based on polarity of a molecule.
The polarity is the property of a molecule which arises due separation of electric charge. Due to this separation of charges molecule exhibit dipole moment which means one end of molecule accommodates slightly positive charge and another end accommodates slightly negative charge.
Fundamentals
The dipole moment for polar molecules is non-zero and non-polar molecule is zero.
In a polar molecule, electronegativity of their atoms are not equal. In a non-polar molecule, electronegativity of their atoms are equal.
Answer:
Part 1
In ![]() , there is a difference between electronegativity of fluorine and bromine atom and due to its square pyramidal structure
, there is a difference between electronegativity of fluorine and bromine atom and due to its square pyramidal structure ![]() is polar molecule.
is polar molecule.

Part 2
In ![]() , there is a difference between electronegativity of oxygen and hydrogen and due to its bent structure
, there is a difference between electronegativity of oxygen and hydrogen and due to its bent structure ![]() is polar molecule.
is polar molecule.
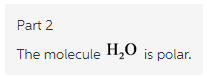
Part 3
In ![]() , although there is a difference between electronegativity of chlorine and boron atom but due to its trigonal planar structure
, although there is a difference between electronegativity of chlorine and boron atom but due to its trigonal planar structure ![]() has zero dipole moment. It is a non-polar molecule.
has zero dipole moment. It is a non-polar molecule.
