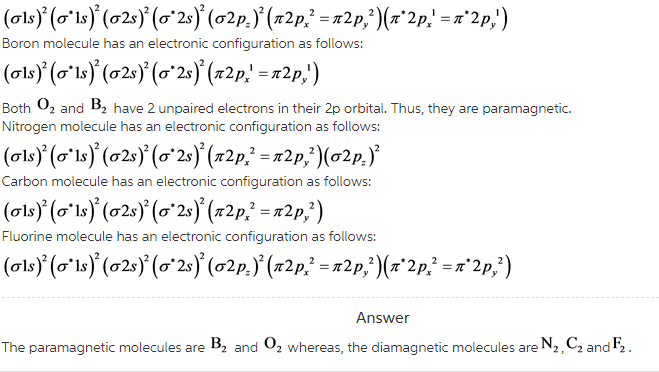Classify these diatomic molecules as diamagnetic or paramagnetic: O2, F2, B2, C2, N2.
Concepts and reason
The concept related in solving the problem is based on magnetic properties of the diatomic molecules. Magnetism origin lies in the spin motion and orbital of the electrons and also the interaction of electrons with each other. Thus, magnetism types are determined on the basis of the behavior of materials in the presence of magnetic fields.
Fundamentals
All the matter is considered to be magnetic. A system’s magnetic moment measures the direction and strength of its magnetism. The materials magnetic behavior is classified into many types like ferromagnetism, paramagnetic, diamagnetism, anti-ferromagnetism and ferrimagnetism.
Answer:
The diamagnetic molecules or materials are those materials, which possess negative and weak susceptibility to the fields that are magnetic. In response to the magnetic field the diamagnetic molecules are repelled. Therefore, there is non-retaining of magnetic properties when, there is removal of external field. Here, there is absence of permanent magnetic moment per atom (net) because there are paired electrons.
The paramagnetic molecules or materials are those which possess positive and small susceptibility to the fields that are magnetic. These materials are attracted to the magnetic fields slightly. Therefore, there is non-retaining of magnetic properties when, there is removal of external field. Here, there are electrons that are unpaired.
Explanation:
The diamagnetic properties and paramagnetic properties of the molecules are determined on the basis of the electrons. It is determined whether the electrons are paired or are unpaired. The molecules with paired electrons are known as diamagnetic whereas, the molecules with unpaired ones are called as paramagnetic molecules.
Explanation:
The diamagnetic properties and paramagnetic properties of the molecules are determined on the basis of electrons. It is determined whether the electrons are paired or are unpaired. The molecules with paired electrons are known as diamagnetic whereas, the molecules with unpaired ones are called as paramagnetic molecules.
Oxygen molecule has an electronic configuration as follows: