Classify the following phase changes by the signs of the system’s ΔH and ΔS.
Answer:
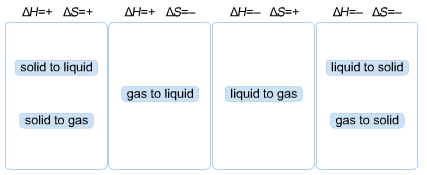
If dH = positive; then dH = Hfinal - Hinitial > 0, i.e. Hfinal > Hinitial, so there must be a change of phase which requires energy; examples: solid to liquid, liquid to gas, solid to gas
If dH negative, then dH = Hfinal - Hinitial < 0, i.e. Hfinal < Hinitial, so there must be a change of phase which releases energy; examples: liquid to solid , gas to liquid, gas to solid
For entropy, recall entropy is related to chaos, so the more excited the state, the more enrtopy
solid < liquid < gas, therefore
Case 1)
positive S + positive H
solid to liquid
solid to gas
liquid to gas
Case 2)
negative S + negative S
gas to liquid
liquid to solid
gas to solid
positive S + negative H
Case 3)
positive S + negative H
Impossible, since you can’t have chaos creation without positive H
Case 4)
negative S + positive H
Impossible, since enthalpy addition will provide chaos