Classify each of the following as an electrophile or a nucleophile.
Concepts and reason
Electrophile is an electron loving and nucleophile is a nucleus loving.
Electron pair acceptors are electrophiles, they have empty orbitals.
Electron pair donors are nucleophiles, they have non-bonding electrons.
Fundamentals
Lewis acid (electrophile) reacts with Lewis base (nucleophile) to form a new covalent bond.
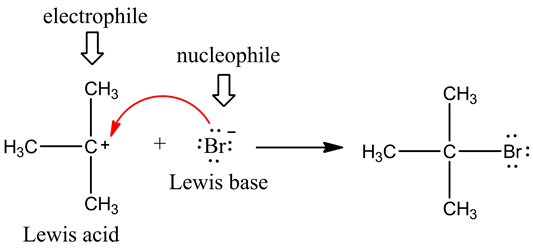
Nucleophile (electron-rich species) reacts with electrophiles (electron-poor ones).
Electrophiles are positively charged species or neutral species with vacant orbitals.
Nucleophiles are negatively charged species or contain lone pairs.
Answer:
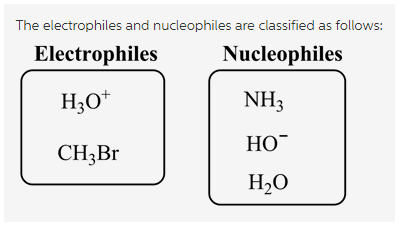
The electrophiles are as follows:
Explanation:
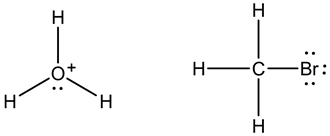
Here, hydronium ion,![]() , is an electrophile because it contains a positive charge on oxygen atom. It accepts electron-pair from other species.
, is an electrophile because it contains a positive charge on oxygen atom. It accepts electron-pair from other species.
Methyl bromide,![]() , is an electrophile because when the heterolytic cleavage occurs between C-Br bond then carbon carries a positive charge, which is an electrophilic center.
, is an electrophile because when the heterolytic cleavage occurs between C-Br bond then carbon carries a positive charge, which is an electrophilic center.
The nucleophiles are as follows:
Explanation:
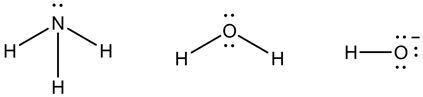
Here, ammonia is a nucleophile because it contains a pair of electrons on nitrogen atom.
Water molecule is a nucleophile because it contains a pair of electrons on oxygen atom.
Hydroxide ion is a nucleophile because it contains a negative charge and has a pair of electrons on oxygen atom.