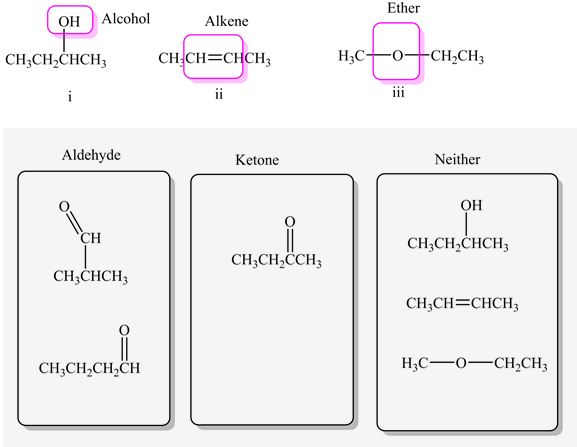
Classify each molecule as an aldehyde, ketone, or neither.
Concepts and reason
The structures of several molecules are given and they need to be identified as aldehyde, ketone or neither. The groups responsible for the characteristic chemical properties of a molecule are known as functional groups.
Fundamentals
The functional groups that are asked in the problem are listed below:
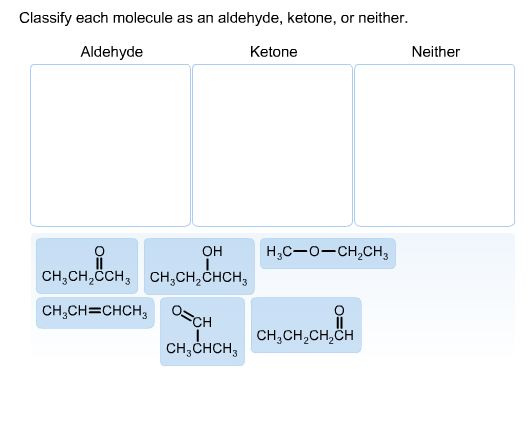
Aldehyde and ketone: Both the groups have a carbonyl group *(C = H)**. When the carbonyl group is attached to one alkyl group, then it is known as aldehyde and when both the groups attached are alkyl groups, then it is known as ketone.
Where R, R’, R” are the different alkyl groups.
Answer:
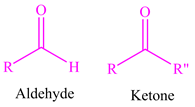
The aldehyde molecules are as follows:
Explanation:
An aldehyde moiety has a carbonyl group (C = H) attached to an alkyl group and hydrogen that is RCHO. Both the above compounds have such a moiety present. Therefore, the functional group present is aldehyde.
The ketone molecules are as follows:

Explanation:
A ketone moiety has a carbonyl group attached to two alkyl groups that is RCOR’. The above molecule has the moiety![]() , in which the carbonyl (C = H) is attached to methyl and methylene group. Therefore, the functional group present is ketone.
, in which the carbonyl (C = H) is attached to methyl and methylene group. Therefore, the functional group present is ketone.
The remaining molecules are neither aldehydes nor ketones.
Explanation:
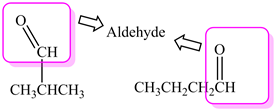
The above molecules are neither aldehydes nor ketones because:
- The molecule (i) has the presence of -OH group which corresponds to the functional group alcohol.
- The molecule (ii) has the presence of C=C group which corresponds to alkene.
- The molecule (iii) has an oxygen single bonded to two alkyl groups which corresponds to the functional group ether.