Carbon monoxide is readily absorbed by ammoniacal cuprous chloride solution but carbon dioxide is not. Explain.
Due to the presence of a lone pair of electrons on carbon in CO, it acts as a Lewis base (or ligand) and thus forms a soluble complex with ammoniacal cuprous chloride solution.
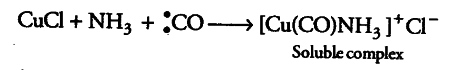
On the other hand,carbondioxide does not act as a Lewis base since it does not have a lone pair of electrons on the carbon atom and hence, does not dissolve in ammoniacal cuprous chloride solution.