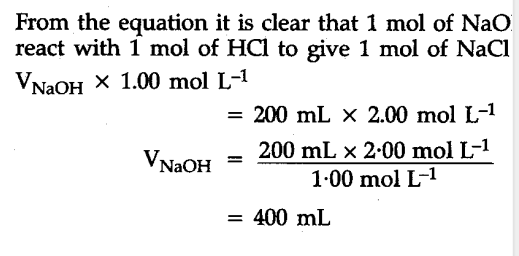
Calculate the volume of 1.00 mol ${{L}^{-1}}$ aqueous sodium hydroxide that is neutralised by 200 mL of 2.00 mol ${{L}^{-1}}$ aqueous hydrochloric acid arid the mass of sodium chloride produced.
Neutralization reaction is :
NaOH(aq) + HCl(aq) -> NaCl(aq) + ${{H}_{2}}$O(I)
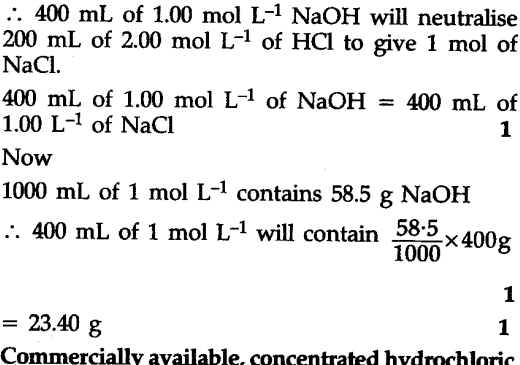
Thankew soo… Much😁