Calculate the value of Kp for the equation
C(s) + CO2(g) <–> 2CO(g) Kp = ?
Given that at a certain temperature
C(s) + 2H2O(g) <–> CO2(g) + 2H2(g) Kp1 = 3.97
H2(g) + CO2(g) <–> H20(g) + CO(g) Kp2 = 0.623
Concepts and reason
An equilibrium constant for a chemical reaction is a certain value of the reaction at equilibrium state, which depends on the temperature at equilibrium. The equilibrium constant for an equilibrium reaction can be expressed in two ways: one is in terms of concentration, the other is in terms of partial pressures.
Fundamentals
The equilibrium constant in terms of partial pressure is represented as. The equilibrium constant can be represented for both homogeneous and heterogeneous equilibria.
Homogeneous equilibria:
The reactants and products are in the same phase.
Example:
![]()
Heterogeneous equilibria:
The reactants and products are in different phases.
![]()
The relationship between two equilibrium constants can be:
![]()
Answer:
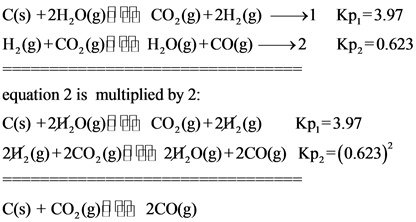
From the given two equations, multiply the second equation by 2 and then cancel the same reactants in both of the equations to get the target equation.

![]()
From the given reactions, there is no change in equation 1. Therefore, there is no change in the value of ![]() . Equation 2 is multiplied by 2. Hence the value of
. Equation 2 is multiplied by 2. Hence the value of ![]() is squared. Then, both equilibrium constant values are multiplied to get the final
is squared. Then, both equilibrium constant values are multiplied to get the final ![]() value.
value.