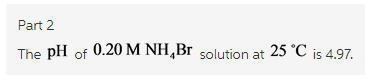-
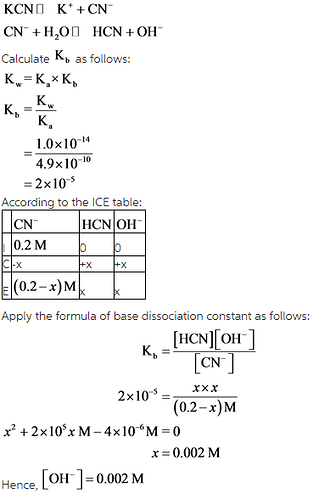
Calculate the pH of a 0.20 M solution of KCN at 25.0 ∘C.
Express the pH numerically using two decimal places.
ph= -
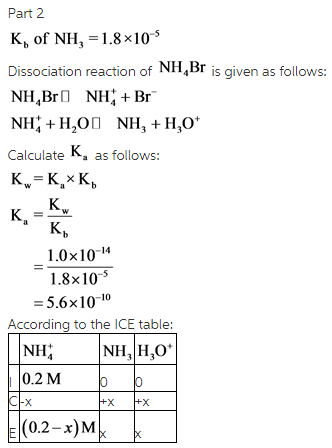
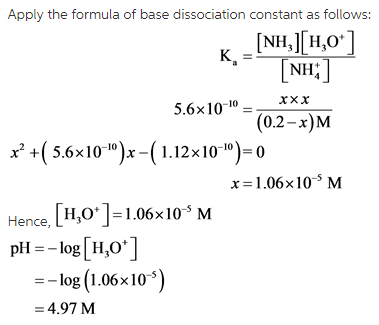
Calculate the pH of a 0.20 M solution of NH4Br at 25.0 ∘C.
Express the pH numerically using two decimal places.
ph=
Answer:
Part 1
![]()
Dissociation reaction of KCN is given as follows: