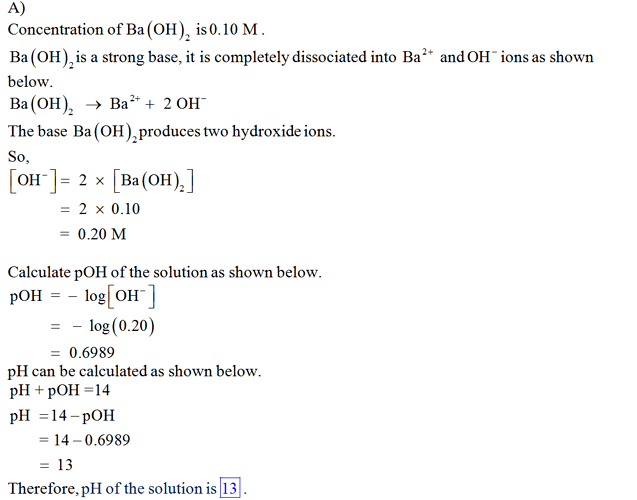
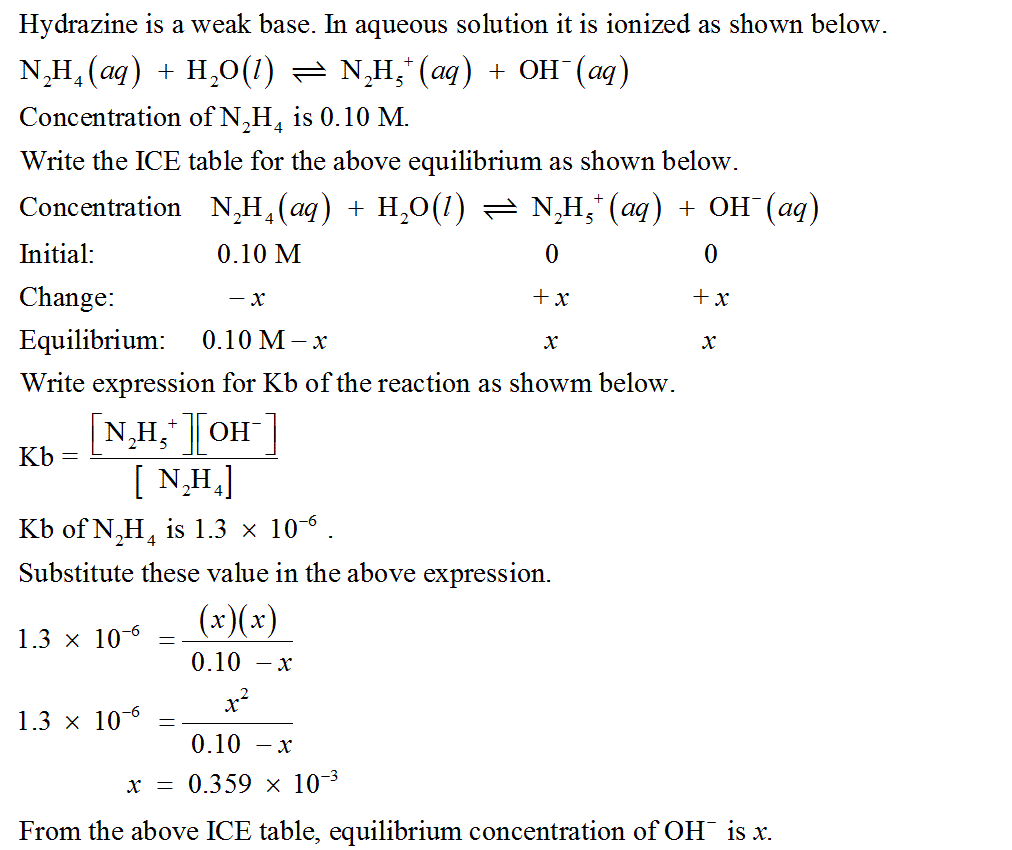A) Calculate the pH of a 0.10 M solution of barium hydroxide, Ba(OH)2. Express your answer numerically using two decimal places.
B) Calculate the pH of a 0.10 M solution of NaOH. Express your answer numerically using two decimal places.
C) Calculate the pH of a 0.10 M solution of hydrazine, N2H4. Kb for hydrazine is 1.3×10−6. Express your answer numerically using two decimal places.
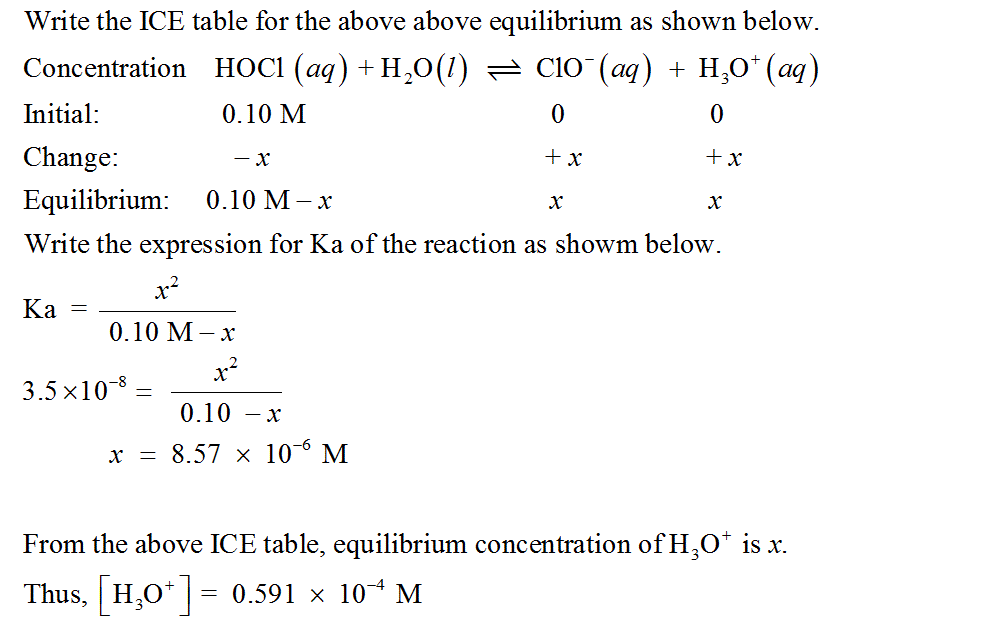
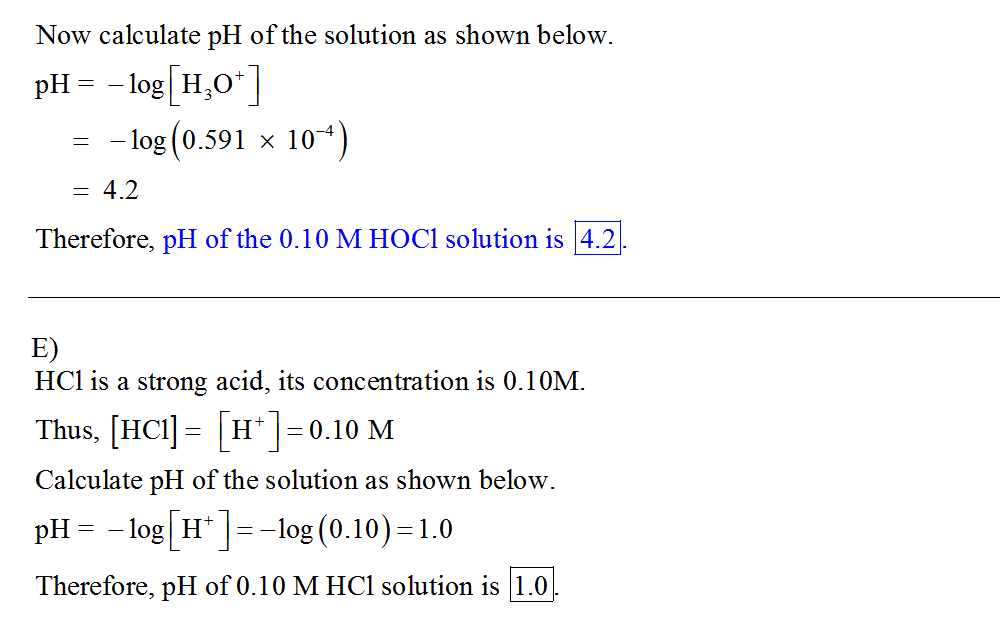
D) Calculate the pH of a 0.10 M solution of hypochlorous acid, HOCl. Ka of HOCl is 3.5×10−8. Express your answer numerically using two decimal places.
E) Calculate the pH of a 0.10 M solution of HCl. Express your answer numerically using two decimal places.
Answer: