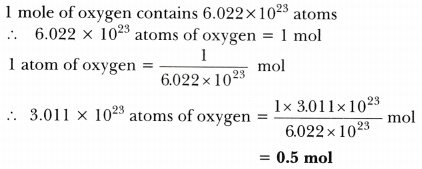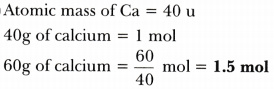Calculate the number of moles present in:
- 3.011 X 10^23 number of oxygen atoms.
- 60 g of calcium
[Given that atomic mass of Ca = 40 u, Avogadro No. = 6.022 X 10^23]
Answer:
Calculate the number of moles present in:
[Given that atomic mass of Ca = 40 u, Avogadro No. = 6.022 X 10^23]
Answer: