- Calculate the number of moles of iron in a sample containing 10 22 atoms of iron.
- Calculate the number of moles in 71g of chlorine. [Atomic mass ofCl= 35.5 u]
- Number of moles of iron
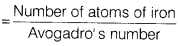
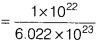
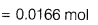
- Molecular mass of
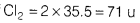
Number of moles of Cl =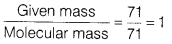
∴ 71 g of Cl2 = 1 moj