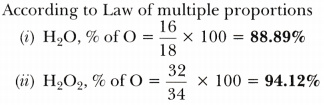Calculate the mass percentage of oxygen present in the following compounds and state the law of chemical combination associated. Given, H = 1, O = 16.
- Water (H20) and
- Hydrogen peroxide (H202)
Answer:
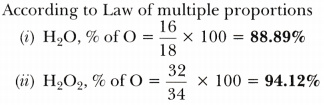
Calculate the mass percentage of oxygen present in the following compounds and state the law of chemical combination associated. Given, H = 1, O = 16.
Answer: