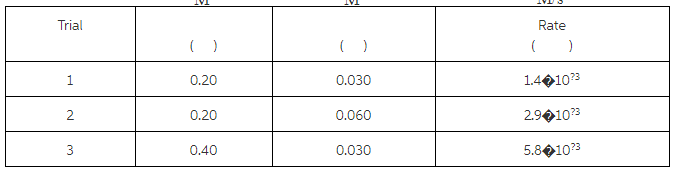
Calculate the initial rate for the formation of C \rm C at 25 ?
A =2, B =1 k[A]^2
strong textConcepts and reason
Rate:
The rate of a chemical reaction is defined as the change in concentration of substance (reactant or product) per unit time. It is expressed in M/S.
Order:
Order of the reaction is a number, which indicates the raise of concentration of the substance.
Significant figures:
The term significant figures refer to the meaningful digits in it.
Fundamentals
Answer:
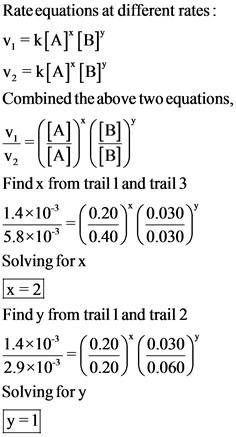
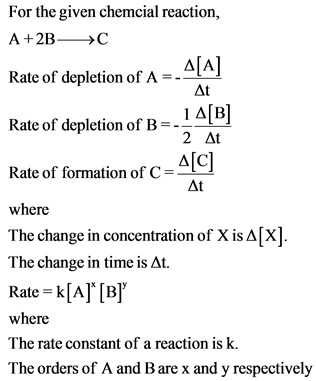
The given three different sets of initial concentrations are used to find the order of each reactant by combining different rate equations. The order of reactant A is 2 and the order of reactant B is 1.
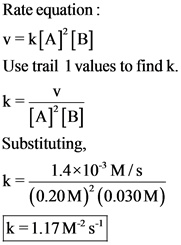
The value of rate constant is obtained by substituting the concentrations of A and B and the initial rate value into the rate equation.
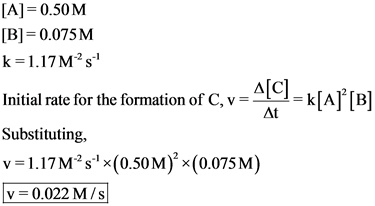
The initial rate for the formation of reactant, C at 25 °C is ![]()
The initial rate for the formation of reactant C is obtained by substituting the concentrations of A and B and the value of rate constant into the rate equation.