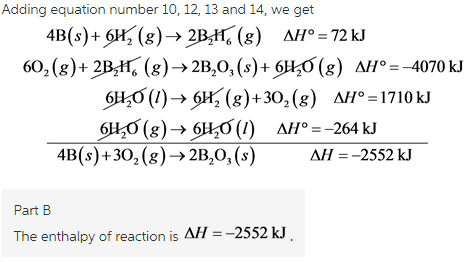Part A
Calculate the enthalpy of the reaction
2NO(g)+O2(g)→2NO2(g)
given the following reactions and enthalpies of formation:
12N2(g)+O2(g)→NO2(g), ΔH∘A=33.2 kJ
12N2(g)+12O2(g)→NO(g), ΔH∘B=90.2 kJ
Express your answer with the appropriate units.
Part B
Calculate the enthalpy of the reaction
4B(s)+3O2(g)→2B2O3(s)
given the following pertinent information:
B2O3(s)+3H2O(g)→3O2(g)+B2H6(g), ΔH∘A=+2035 kJ
2B(s)+3H2(g)→B2H6(g), ΔH∘B=+36 kJ
H2(g)+12O2(g)→H2O(l), ΔH∘C=−285 kJ
H2O(l)→H2O(g), ΔH∘D=+44 kJ
Express your answer with the appropriate units.
Concepts and reason
An equation which contains all the reactants and products with their physical states mentioned in brackets and enthalpy of reaction is termed as thermochemical equation.
The enthalpy of a reaction is the change in energy during the reaction at given temperature and pressure.
The enthalpy of given reaction is calculated using Hess’s law by taking help of the reaction enthalpies of other given reactions.
Fundamentals
Hess’s law states that the total enthalpy change for a reaction during the complete course of time is same irrespective of the path it follows.
If a reaction is made up of more than one step, the overall enthalpy of the final reaction is the summation of individual enthalpies of each step.
The thermochemical equation follows some simple set of rules to calculated the enthalpy of a reaction:
- If a reaction is reversed, the enthalpy change sign is also reversed.
- If a reaction is multiplied/divided by a constant, the enthalpy is also multiplied/divided with that same number.
Answer:
Part A
The given reactions are as follows:
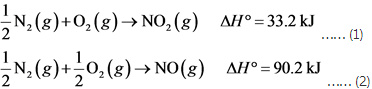
Equation number 2 is reversed and multiplied by 2 to get,
![]()
The reaction for which enthalpy is to be determined is,
![]()
Equation number 1 is multiplied by 2 to get,
![]()
Adding equation number 3 and 5, we get
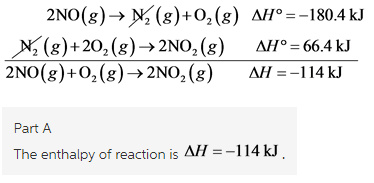
Part B
The given reactions are as follows:
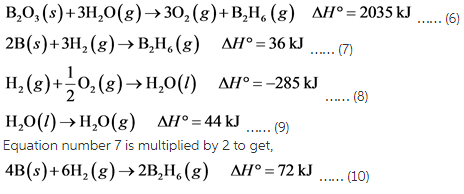
The reaction for which enthalpy is to be determined is,
![]()
From the equation, the required reaction contains 4 moles of B as a reactant, therefore, equation number 7 is multiplied by 2.
Equation number 6 is multiplied by 2 and reversed to get,
![]()
Equation number 8 is multiplied by 6 and reversed to get,
![]()
Equation number 9 is multiplied by 6 and reversed to get,
![]()