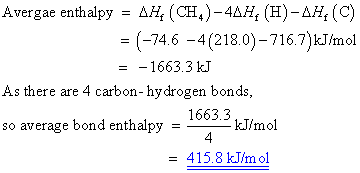Calculate the average molar bond enthalpy of the carbon-hydrogen bond in a CH4 molecule.
Given that
(Delta)Hf [H(g)]= 218.0 kj/mol
(Delta)Hf [C(g)]= 716.7 kJ/mol
(Delta)Hf [CH4(g)]= -74.6 kJ/mol
Answer:
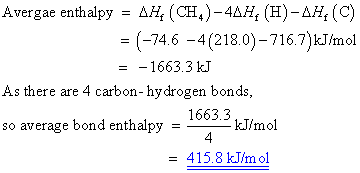
Calculate the average molar bond enthalpy of the carbon-hydrogen bond in a CH4 molecule.
Given that
(Delta)Hf [H(g)]= 218.0 kj/mol
(Delta)Hf [C(g)]= 716.7 kJ/mol
(Delta)Hf [CH4(g)]= -74.6 kJ/mol
Answer: