calculate the average molar bond enthalpy of the carbon-bromine bond in a CBr4 molecule.
given thay :
deltaH= Br(g)= 111.9 kj
dH= C(g)= 716.7 jk
dH= CBr4(g)= 29.4
Concepts and reason
- The thermodynamic quantity “enthalpy” is state function that depends on the initial and final states of the system.
- The enthalpy is measured when a system is at the constant pressure; the heat released or absorbed is equal to the change in enthalpy.
- The bond enthalpy is also known as the bond energy, and it is defined as the energy required for breaking the chemical bonds in a molecule.
- The average molar bond enthalpy can be calculated by dividing the bond enthalpy with total number of bonds present in a molecule
Fundamentals
The arbitrary reaction is given below:
![]()
The formulae to calculate the enthalpy of reaction is given below:
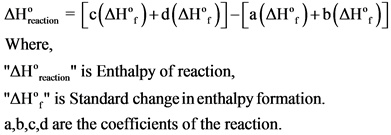
Answer:
Consider the formation of ![]() molecule from its elements.
molecule from its elements.
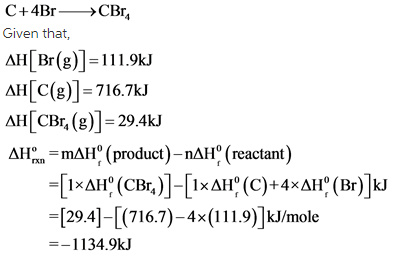
The standard enthalpies of product such as Carbon tetrabromide ![]() and the standard enthalpies of reactants such as carbon ( C) and four bromine atoms (Br) added. The enthalpy of reaction is calculated by subtracting the standard enthalpies of reactants from the enthalpies of the formation of products.
and the standard enthalpies of reactants such as carbon ( C) and four bromine atoms (Br) added. The enthalpy of reaction is calculated by subtracting the standard enthalpies of reactants from the enthalpies of the formation of products.
The number of carbon-bromine bonds in ![]() molecule is four.
molecule is four.
Therefore, the average bond enthalpy is given below:

Therefore, the average molar bond enthalpy is 283.725kJ.
The average molar bond enthalpy of the carbon-bromine bond in a ![]() molecule is 283.725kJ.
molecule is 283.725kJ.