Boron fluoride exists as $BF_{ 3 }$ but boron hydride doesn’t exist as $BH_{ 3 }$. Give reason. In which form does it exist? Explain its structure
Boron fluoride exists as BF_{ 3 }. Due to extensive pit-pn back bonding between B and fluoride ions, BF_{ 3 } cannot form the dimeric molecule. On the other hand, boron hydride exists. This is due to the feet that hydrogen atom in BH_{ 3 } has no electron to form p$\pi$-p$\pi$ back bonding. Thus, boron possesses incomplete octet and BH_{ 3 } is dimerised to form molecule with covalent and three centre bond.
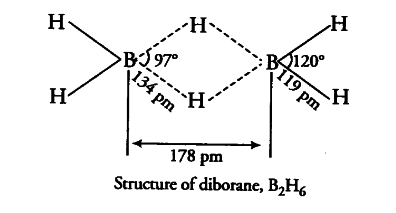
the four terminal hydrogen atoms and the two boron atoms lie in one plane. Above and below this plane there are two bridging H-atoms. The four terminal B—H bonds are regular while the two bridge (B—H—B) bonds are three centre-two electron bonds.