Aryl halides are extremely less reactive towards nucleophilic substitution. Predict and explain the order of reactivity of the following compounds towards nucleophilic substitution.
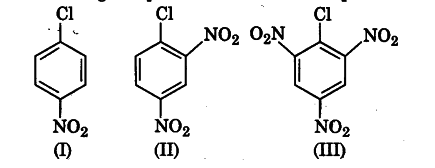
Aryl halides are extremely less reactive towards nucleophilic substitution. Predict and explain the order of reactivity of the following compounds towards nucleophilic substitution.

Aryl halides are less reactive towards nucleophilic substitution reaction. Presence of electron withdrawing group at o- and p-position increases the stability of intermediates and hence increases the reactivity of aryl halides towards nucleophilic substitution reaction.
Now, more the number of EWG at o- and p-position, higher will be the reactivity of aryl halide. Compound (III) has three EWG so, it is most reactive and compound (I) has only one EWG, so it is least reactive. So, the order of reactivity is (I) < (II) < (III).