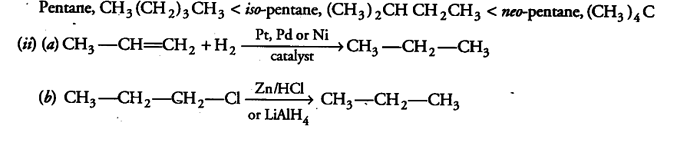
(i) Arrange the three isomeric pentanes in order of increasing stability at room temperature,
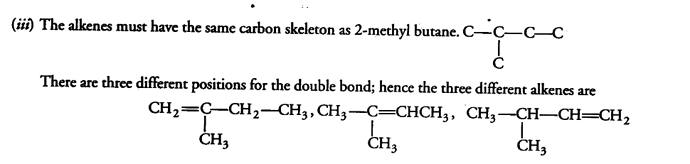
(ii) Give a method of preparation of propane from (a) an alkene (6) an alkyl halide.
(iii) Write the structure of all the alkenes that can be hydrogenated to form 2-methyl butane, (zv) Why is light or heat necessary to initiate the chlorination reaction?