Arrange the following in order of decreasing masses:
- 10^23 molecules of C02 gas
- 0.1 g atom of silver
- 1 gram of carbon
- 0.1 mole of H2S04
- 10^23 atoms of calcium.
(Given Atomic masses: Ag = 108 u, S = 32 u, N = 14 u, Ca = 40 u)
Answer:
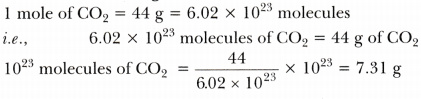
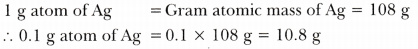
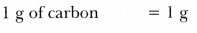
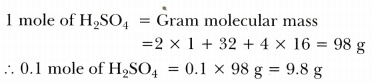
-
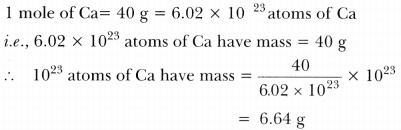
Thus, masses in the decreasing order are:
0.1 g atom of Ag >0.1 mole of H2S04 > 10^23 molecules of C02 > 10^23 atoms of Ca > 1 g of carbon