Nitro group shows —I and — R- effect as follows
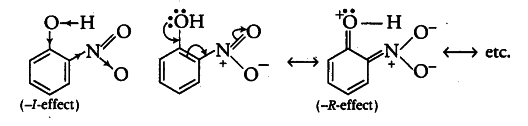
Due to the -I-and -R-effect of o-nitrophenol, it is a stronger acid than phenol thus making the release of {{H}^{+}} ion from O — H bond easier. —I- and —R-effect increases the acidic strength by increasing the polarity of —OH bond. On the other hand, —$CH _{ 3 }$ group in o-cresol produces +I-effect which decreases the polarity due to increase in electron density on —OH bond. So, o-cresol is a weaker acid than phenol. Thus, the correct order is o-cresol < phenol < o-nitrophtenol.