Anisole reacts with HI to give phenol and methyl iodide and not iodobenzene and methylalchohol. Give reason and explain the reaction, with mechanism.
Anisole on protonation forms methylphenyl oxonium ion.
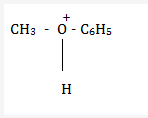
The bond between O-CH3 is weaker than the bond between O-C6H5.
This is because of the partial double bond character between oxygen and carbon of the benzene ring.
Hence the I- ion attacks and breaks the O-CH3 bond to give CH3I.
The phenols formed do not react with halides since nucleophilic substitution on aromatic ring is difficult.