An ester has the molecular formula C4H802. Write its structural formula. What happens when this ester is heated in the presence of sodium hydroxide solution? Write the balanced chemical equation for the reaction and name the products. What is a saponification reaction?
Answer:
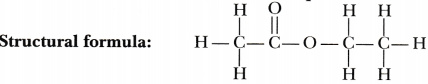
When this ester is heated in the presence of sodium hydroxide solution, it changes into an alcohol and a carboxylic acid
![]()
Products: Ethanol and ethanoic acid
Saponification: Reaction of an ester with an acid or a base to give an alcohol and a carboxylic acid. This reaction is known as saponification because it is used in the preparation of soap.